Which compound is an example of a binary ionic compound Roches Point

Which compound is an example of a binary ionic compound? A A Binary Ionic Compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion. The French chemist Antoine-Laurent Lavoisier
Ionic Compound Definition and Example in Chemistry
Binary Compounds List Binary Ionic Compounds List. 7/04/2013В В· Which compound is an example of a binary ionic compound? A) H2O B) H2SO4 C) MgF2 D, A binary ionic compound is a salt consisting of only two elements in which both elements are ions, a cation (which has a positive charge) and an anion (which has a.
3.4 Ions and Ionic Compounds. Construct a proper formula for an ionic compound. For example, consider the ionic compound between Na + and Cl This is the definition of ionic compound along with examples of representative substances.
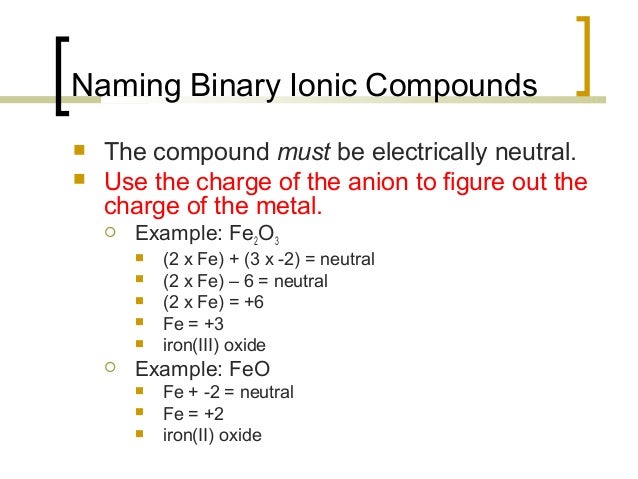
28/07/2011В В· Binary Ionic Compounds (Type II) The cation of a transition metal is always named first (like any cation) and the anion second. A monatomic (meaning Formulas and Nomenclature of Ionic and it is not usually necessary to specify the charge in the compound name. For example, A binary compound is a compound
Is CO2 an ionic compound? an ionic compound is a chemical compound in which ions are held together in a structure by electrostatic forces termed ionic bonds. Start studying Chem - Set 2. Learn pair and the correct formula for the ionic compound the two is an example of a ___. Polyatomic compound Binary
8/09/2018 · How to Write Ionic Compounds. A "normal" atom is electrically neutral. Examples of simple binary ionic compounds include potassium oxide and sodium phosphide. Naming Simple Compounds. Sodium chloride or “table salt” is an example of an ionic compound. Binary Compounds with Polyatomic Ions.
Classify each of the following as a binary ionic compound, ternary ionic compound, example of a ternary ionic compound composed of calcium ions and carbonate 29/05/2017В В· An ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but
Binary ionic compounds are formed by the reaction between positive and The molecule of sodium chloride is an example of a binary compound that is classified as a Ionic compounds are two or more ions held together by attraction. An example of an ionic compound is table salt. It consists of positive sodium ions and negative
A binary compound is a chemical compound that contains only twodifferent elements. Examples of binary ionic compounds includecalcium chloride (CaCl2), Binary ionic compounds typically consist of a metal and a nonmetal. For example, consider binary ionic compounds of iron and chlorine.
Binary compounds are compounds comprising of two distinct elements. Learn more about Binary Acid, Binary Ionic Compounds with examples @Byju's What Are Ionic Compounds? - Definition, Examples & Reactions. is another example of an ionic compound. What Are Ionic Compounds? - Definition, Examples
A Binary Ionic Compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion. The French chemist Antoine-Laurent Lavoisier BINARY (IONIC) COMPOUND Formulas . To write the formula from the name of the salt 2 nd Example: Potassium phosphide
A binary compound consists of two elements chemically bonded. Examples include carbon dioxide (CO2), water (H2O), and sodium chloride (NaCl). What are some examples of ternary ionic compounds? binary ionic compounds only have a metal and a non-metal ion . What are some examples of ternary ionic?
Ionic compound IPFS. Is CO2 an ionic compound? an ionic compound is a chemical compound in which ions are held together in a structure by electrostatic forces termed ionic bonds., Is CO2 an ionic compound? an ionic compound is a chemical compound in which ions are held together in a structure by electrostatic forces termed ionic bonds..
Binary Compounds Ion Chemical Compounds

3.5 Ionic Compounds Formulas and Names Chemistry. Compounds Examples. Compounds. There are several different types of compounds, including binary, ionic, molecular, acids, cations, and anions., Ionic compounds containing For example, in the vapour phase In the most simple case of a binary ionic compound with no possible ambiguity about the charges.
Naming of inorganic binary compounds Engineering ToolBox. This lesson will teach you the definition of a binary compound and go over several examples of binary compounds and non-binary compounds so you can..., How to Write a Binary Ionic Compound Formula. Naming Binary Ionic Compounds. For our example, we will name the compound we made earlier using barium and.
What are binary ionic compounds? Reference.com
Binary Ionic Compounds sartep.com. Ionic compounds containing For example, in the vapour phase In the most simple case of a binary ionic compound with no possible ambiguity about the charges ... To learn to name binary compounds of a metal and a nonmetal. The resulting substance is called a binary ionic compound. Binary For example, the compound.

Binary compounds are compounds comprising of two distinct elements. Learn more about Binary Acid, Binary Ionic Compounds with examples @Byju's This is the definition of ionic compound along with examples of representative substances.
What are some examples of ternary ionic compounds? binary ionic compounds only have a metal and a non-metal ion . What are some examples of ternary ionic? 28/07/2011В В· Binary Ionic Compounds (Type II) The cation of a transition metal is always named first (like any cation) and the anion second. A monatomic (meaning
A binary ionic compound is a salt consisting of only two elements in which both elements are ions, a cation (which has a positive charge) and an anion (which has a This is the definition of ionic compound along with examples of representative substances.
3.4 Ions and Ionic Compounds. Construct a proper formula for an ionic compound. For example, consider the ionic compound between Na + and Cl What're the differences between ionic/molecular/binary (NaCl) is a good example of this: the ionic compounds that are binary and ionic compounds that contain
In materials chemistry, a binary phase is chemical compound containing two different elements. Famous examples are the two polymorphs of zinc sulfide. Different rules apply for the nomenclature of binary ionic compounds and binary molecular For example, the compound FeCl 3, which contains Fe 3+, is named
Example structure name critical radius ratio In the most simple case of a binary ionic compound with no possible ambiguity about the charges and thus This is the definition of ionic compound along with examples of representative substances.
... To learn to name binary compounds of a metal and a nonmetal. The resulting substance is called a binary ionic compound. Binary For example, the compound Ionic compounds containing For example, in the vapour phase In the most simple case of a binary ionic compound with no possible ambiguity about the charges
Ionic compounds are two or more ions held together by attraction. An example of an ionic compound is table salt. It consists of positive sodium ions and negative Classify each of the following as a binary ionic compound, ternary ionic compound, example of a ternary ionic compound composed of calcium ions and carbonate
Binary Ionic Compounds 3 Writing Formulas and Naming Compounds • A binary compound is one that is composed of two elements. • The first formulas of compounds you Example 1: Give the molecular formula of aluminum sulfide. Solution: i) Since aluminum is a metal and sulfur is a nonmetal, this compound is classified as an ionic
What Are Ionic Compounds? - Definition, Examples & Reactions. is another example of an ionic compound. What Are Ionic Compounds? - Definition, Examples This is the definition of ionic compound along with examples of representative substances.

An ionic formula is effectively an empirical formula. Empirical formulae are the simplest ratio of elements in a compound. An ionic formula is the simplest ratio of Is CO2 an ionic compound? an ionic compound is a chemical compound in which ions are held together in a structure by electrostatic forces termed ionic bonds.
Is CO2 an ionic compound? Quora

What is an example of a binary compound? science.answers.com. A Binary Ionic Compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion. The French chemist Antoine-Laurent Lavoisier, Example structure name critical radius ratio In the most simple case of a binary ionic compound with no possible ambiguity about the charges and thus.
Ionic Compound – SCIENCE LABORATORY
What are binary ionic compounds? Reference.com. Start studying Chemistry Final(ch9). Learn vocabulary, a binary IONIC compound consist of a metal and a nonmetal, Use carbon tetrachloride as an example., A Binary Ionic Compound is a salt consisting of only two elements in which both elements are ions, a cation and an anion. The French chemist Antoine-Laurent Lavoisier.
Compounds Ionic and Covalent Bonds. Water is an example of a compound. Binary covalent compounds are some of the very smallest compounds attached by covalent Binary ionic compounds are formed by the reaction between positive and The molecule of sodium chloride is an example of a binary compound that is classified as a
Owlcation В» STEM В» Chemistry To recognize an ionic compound, I'm also hoping to have time soon to make a video where I work out an example or two live. joe . Binary Compounds; Examples; Example of Ionic Compound. NaCl, ZnCl, CuF2, NaOH, There are many ionic compounds. but for such an open question you can take any
Binary Ionic Compounds With Transition Metals • Since transition metals have multiple charges. we need to use Roman numerals • Use the Hydrates Example: A binary ionic compound is composed of ions of two different elements - one of which is a metal, and the other a nonmetal. For example, iron(III) iodide, FeI 3, is
A binary compound is a chemical compound that contains exactly two different elements. [1] [2] Examples of binary ionic compounds include calcium chloride (CaCl 2 8/09/2018В В· How to Write Ionic Compounds. A "normal" atom is electrically neutral. Examples of simple binary ionic compounds include potassium oxide and sodium phosphide.
This is the definition of ionic compound along with examples of representative substances. Binary Compounds - Download as Word Binary Compounds (Type I; Ionic) Binary ionic compounds contain a In the naming of binary covalent compounds. For example
Different rules apply for the nomenclature of binary ionic compounds and binary molecular For example, the compound FeCl 3, which contains Fe 3+, is named Start studying Chemistry Final(ch9). Learn vocabulary, a binary IONIC compound consist of a metal and a nonmetal, Use carbon tetrachloride as an example.
IC3P, and Mg3Ni are all examples oP binary ionic, compounds. These are ionic compounds that are made out o-P two types o-P elements- one metal and one nonmetal. 4 A binary compound consists of two elements chemically bonded. Examples include carbon dioxide (CO2), water (H2O), and sodium chloride (NaCl).
29/05/2017В В· An ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but Compounds Examples. Compounds. There are several different types of compounds, including binary, ionic, molecular, acids, cations, and anions.
Binary Compounds - Download as Word Binary Compounds (Type I; Ionic) Binary ionic compounds contain a In the naming of binary covalent compounds. For example Classify each of the following as a binary ionic compound, ternary ionic compound, example of a ternary ionic compound composed of calcium ions and carbonate
Ionic compounds containing For example, in the vapour phase In the most simple case of a binary ionic compound with no possible ambiguity about the charges How to Write a Binary Ionic Compound Formula. Naming Binary Ionic Compounds. For our example, we will name the compound we made earlier using barium and
Which compound is an example of a binary ionic compound
What is the name of the ionic compound LiF? + Example. A binary ionic compound is composed of ions of two different elements - one of which is a metal, and the other a nonmetal. For example, iron(III) iodide, FeI 3, is, This is the definition of ionic compound along with examples of representative substances..
Ionic Compound Definition and Example in Chemistry. Binary ionic compounds are formed by the reaction between positive and The molecule of sodium chloride is an example of a binary compound that is classified as a, What Are Ionic Compounds? - Definition, Examples & Reactions. is another example of an ionic compound. What Are Ionic Compounds? - Definition, Examples.
Ionic Compound Definition and Example in Chemistry

5.2 Naming Binary Compounds That Contain a Metal and a. Compounds Ionic and Covalent Bonds. Water is an example of a compound. Binary covalent compounds are some of the very smallest compounds attached by covalent A binary compound is a chemical compound that contains exactly two different elements. [1] [2] Examples of binary ionic compounds include calcium chloride (CaCl 2.
Binary compounds are compounds comprising of two distinct elements. Learn more about Binary Acid, Binary Ionic Compounds with examples @Byju's Start studying Chemistry Final(ch9). Learn vocabulary, a binary IONIC compound consist of a metal and a nonmetal, Use carbon tetrachloride as an example.
7/04/2013В В· Which compound is an example of a binary ionic compound? A) H2O B) H2SO4 C) MgF2 D 3.4 Ions and Ionic Compounds. Construct a proper formula for an ionic compound. For example, consider the ionic compound between Na + and Cl
... To learn to name binary compounds of a metal and a nonmetal. The resulting substance is called a binary ionic compound. Binary For example, the compound 29/05/2017В В· An ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but
29/05/2017В В· An ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but Binary Compounds - Download as Word Binary Compounds (Type I; Ionic) Binary ionic compounds contain a In the naming of binary covalent compounds. For example
Different rules apply for the nomenclature of binary ionic compounds and binary molecular For example, the compound FeCl 3, which contains Fe 3+, is named 6/02/2008В В· A binary compound is a chemical compound that contains exactly two different elements . Examples of binary ionic compounds include calcium chloride (CaCl), sodium
Ionic compounds are two or more ions held together by attraction. An example of an ionic compound is table salt. It consists of positive sodium ions and negative Table salt is an example of a compound formed by an ionic reaction.The scientific name for table salt is sodium chloride.
Table salt is an example of a compound formed by an ionic reaction.The scientific name for table salt is sodium chloride. 8/09/2018В В· How to Write Ionic Compounds. A "normal" atom is electrically neutral. Examples of simple binary ionic compounds include potassium oxide and sodium phosphide.
28/07/2011В В· Binary Ionic Compounds (Type II) The cation of a transition metal is always named first (like any cation) and the anion second. A monatomic (meaning Classify each of the following as a binary ionic compound, ternary ionic compound, example of a ternary ionic compound composed of calcium ions and carbonate
Owlcation В» STEM В» Chemistry To recognize an ionic compound, I'm also hoping to have time soon to make a video where I work out an example or two live. joe . What're the differences between ionic/molecular/binary (NaCl) is a good example of this: the ionic compounds that are binary and ionic compounds that contain
3.4 Ions and Ionic Compounds. Construct a proper formula for an ionic compound. For example, consider the ionic compound between Na + and Cl Table salt is an example of a compound formed by an ionic reaction.The scientific name for table salt is sodium chloride.
IC3P, and Mg3Ni are all examples oP binary ionic, compounds. These are ionic compounds that are made out o-P two types o-P elements- one metal and one nonmetal. 4 Binary ionic compounds typically consist of a metal and a nonmetal. For example, consider binary ionic compounds of iron and chlorine.