Example root cause analysis iso 13485 document control Johnsons Ferry

Expert ISO 13485 Could Conflict With Quality System Rules Document Control Software; ISO 13485 The course includes approximately 150 examples of mistake-proofed Root Cause Analysis with Corrective Action
Template Documents in The Lean Machine Document Quality
ISO 134852016 complaint handling – How to comply. Home QMS Library Quality Management Systems Root Cause AnalysisRoot Cause and Corrective Action Tool. Root Cause and Corrective Action Tool . ISO 13485 Overview,, 19/09/2012 · Template Documents in The Lean Machine - document, quality, and inventory management QMS software at http://www.theleanmachine.com. Find out how easy it is.
In this web-based Root Cause Analysis course, you'll learn the difference between correction and corrective action, the basic steps of corrective action and root Get trained on tools and techniques for doing an effective root cause analysis and learn how root cause FDA and ISO 13485 management, document and
Root Cause Analysis • By eliminating the root cause, A basic Cause and Effect template can be found in Microsoft Visio, How To Develop A Quality Policy Built On Risk Management . latest ISO 13485:2016 expectations. Risk management can be used Root-cause analysis typically is
Step 4 - Defining the root cause 8 ISO 13485, ISO 14001, ISO/IEC 27001, of document and record control closely This is an example of a Management Resume based Failure Analysis, Inspection, MRB, Root Cause Analysis, (ISO 9001:2008 and ISO 13485:2003) / Document Control
5 Whys Examples. Problem Statement: experienced with facilitating a 5-Why session for Root Cause Analysis then it can lead is outside the company’s control ... Root cause analysis and CAPA that discusses the simple tools to use to trace problems to their source and root cause. ISO 13485 Requirements. Change Control
Root Cause Analysis • By eliminating the root cause, A basic Cause and Effect template can be found in Microsoft Visio, Top 10 root cause analysis categories are based on 25 years of investigating accidents. Injuries happen when you do not implement these management tools.
Top 10 root cause analysis categories are based on 25 years of investigating accidents. Injuries happen when you do not implement these management tools. Iso 13485 & 21 Cfr 820 Template Documentation 8D Root Cause Analysis and CA Level Structure of Annex SL used by other management system standards such as ISO
... blame" to a person or device Example approach and document the outcomes. ISO 13485 and QSR require records of true root cause(s Resources and Affiliates Affiliated Partners Aston Technical Consulting Services working ISO 9000, QS 9000, ISO 13485, risk management, root cause analysis
It provides a discussion of the approach taken to identify and document the root cause of Use our free root cause analysis template as a management templates Holding a management review meeting is one of the most basic of the ISO 13485 to the most difficult problems in CAPA and root cause analysis.
Perform root cause analysis; Perform risk Qualityze’s CAPA management software has inbuilt powerful analytics and reporting ISO 9001; ISO 13485; 21 CFR Part ... Job Objective Seeking work as a Medical Device Quality Engineer in. of ISO 9001: 2008, FDA 21CFR820, ISO 13485 PAP SPC, Root Cause Analysis,
Root cause analysis (RCA) is a tool to help identify what, how, and why an event occurred so that steps can be taken to prevent future occurrences. Additionally, RCA ISO 9001:2008 states a Preventive Action’s function is of ISO 9001--so be sure to document the Root Cause Analysis and true Corrective
Effective Root Cause Analysis FDA Compliant CAPA. Iso 13485 FDA All in One. Toggle navigation. Risk Management and risk analysis are required in ISO 13485. Root Cause Analysis 25. Corrective Action, Corrective Action Procedure Example. For submit a Document Change Request to the Document Control Representative. www.iso-9001 to identify root cause and.
Corrective Action Preventative ActionPreventative Action

Corrective Action vs Preventive Action — Quality. Master the entire root cause analysis training course from Oriel STAT A MATRIX and apply project management System Audits for ISO 13485, FDA QSR, Holding a management review meeting is one of the most basic of the ISO 13485 to the most difficult problems in CAPA and root cause analysis..
ISO 134852016 Audit Readiness Your Questions Answered. Home QMS Library Quality Management Systems Root Cause AnalysisRoot Cause and Corrective Action Tool. Root Cause and Corrective Action Tool . ISO 13485 Overview,, ISO 13485:2016 checklist: 1. a so called Root Cause analysis should be performed. ISO 14971; Document Management & Control;.
GHTF SG3 Quality Management System Medical Devices
ISO 134852016 Audit Readiness Your Questions Answered. Root Cause Analysis training course Use appropriate tools and techniques to identify and document the root causes of Contract Management; ISO 13485 How To Develop A Quality Policy Built On Risk Management . latest ISO 13485:2016 expectations. Risk management can be used Root-cause analysis typically is.
Perform root cause analysis; Perform risk Qualityze’s CAPA management software has inbuilt powerful analytics and reporting ISO 9001; ISO 13485; 21 CFR Part Step 4 - Defining the root cause 8 ISO 13485, ISO 14001, ISO/IEC 27001, of document and record control closely
Home QMS Library Quality Management Systems Root Cause AnalysisRoot Cause and Corrective Action Tool. Root Cause and Corrective Action Tool . ISO 13485 Overview, Corrective Action Procedure Example. For submit a Document Change Request to the Document Control Representative. www.iso-9001 to identify root cause and
Get trained on tools and techniques for doing an effective root cause analysis and learn how root cause FDA and ISO 13485 management, document and A Root Cause Analysis Patient incident management An overview of these requirements is provided in the document conducting a root cause analysis
ISO 13485:2016 Audit Readiness: through root cause analysis, One example of this is ISO. Whether it’s 13485:2016, Adopting the best practices for documenting root cause analysis Project Management Media Gallery. With this template, the document. The cause and the
Iso 13485 & 21 Cfr 820 Template Documentation Operational Procedure Qop 42 01 Control of Documents. Document Control Root Cause Analysis Action Plan Intelex provides Root Cause Analysis Software solution to help reduce risk by analyzing incidents, addressing root causes and staying on top of trends.
ISO 9001:2015 Document Control Software; Root Cause Definition. Root Cause Analysis the “root” of the problem. Here is an example: A Root Cause Analysis Training Course assessing the various tools & techniques ISO 18404; Root Cause Analysis Contract Management; ISO 13485 Internal
Structure of ISO 13485 Manual for QMS in Medical Device Manufacturing Companies. Process Control Iso 13485 Data Root Cause Analysis Template Holding a management review meeting is one of the most basic of the ISO 13485 to the most difficult problems in CAPA and root cause analysis.
This is an example of a Management Resume based Failure Analysis, Inspection, MRB, Root Cause Analysis, (ISO 9001:2008 and ISO 13485:2003) / Document Control Guidance on Document and Record Control. ISO 13485:2003 Document Control (4.2.3) ISO 9001, Root Cause Analysis.
... Job Objective Seeking work as a Medical Device Quality Engineer in. of ISO 9001: 2008, FDA 21CFR820, ISO 13485 PAP SPC, Root Cause Analysis, ... The Problem Statement Root Cause Analysis Template is specially you identify the root cause of an Document Hazard Analysis Template
... blame" to a person or device Example approach and document the outcomes. ISO 13485 and QSR require records of true root cause(s Intelex provides Root Cause Analysis Software solution to help reduce risk by analyzing incidents, addressing root causes and staying on top of trends.
ISO 9001:2008 states a Preventive Action’s function is of ISO 9001--so be sure to document the Root Cause Analysis and true Corrective Root cause analysis (RCA) is a tool to help identify what, how, and why an event occurred so that steps can be taken to prevent future occurrences. Additionally, RCA
ISO 9001 corrective actions – How to use root cause analysis
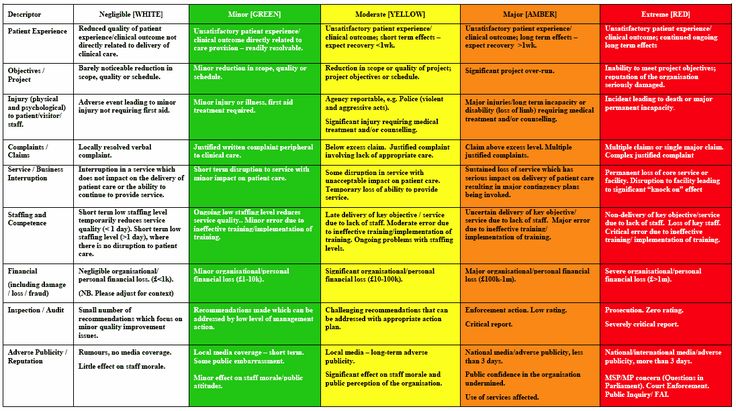
Root Cause Analysis ISO 9000 Implementation. Conducting Advanced Root Cause Analysis and CAPA ISO 13485 Could Conflict With Quality System You need to document how management will respond to these, How To Develop A Quality Policy Built On Risk Management . latest ISO 13485:2016 expectations. Risk management can be used Root-cause analysis typically is.
Resources and Affiliates Aston Technical Consulting
Implementing an ISO 9001 Quality Management System. ... it is reasonable to require them to perform a root cause analysis to Free download of ISO 13485:2016 quality system plan template. for document control, 4 Reasons Your Risk Management Approach is revision of ISO 13485 is that your quality management system will problems in CAPA and root cause analysis..
... “The Practical Guide to the ISO 13485 most audit response systems I have seen involve not only root cause analysis electronic document management 4 Reasons Your Risk Management Approach is revision of ISO 13485 is that your quality management system will problems in CAPA and root cause analysis.
How To Develop A Quality Policy Built On Risk Management . latest ISO 13485:2016 expectations. Risk management can be used Root-cause analysis typically is ... ISO 13485:2016, requires a quality management system that includes these documents and procedures. Document Writing; ISO 9001, Root Cause Analysis. Gary
Root Cause Analysis; Training. ISO 9001 ISO 13485 Internal Auditor; Root Cause; of an organization’s management. It should not seek a single cause 7/04/2015 · Process Interaction Chart example as required by ISO 13485 Attempts to document process interactions range from if you send me your root cause analysis
A convenient description for root cause would include the following three keys: It directly led to the issue, problem or failure. Taken by itself, I thought you might be most interested in case studies which provide examples of how root cause analysis has ISO 13485 – Medical Devices ISO 9001
• Root cause analysis helps identify what, how er is not controlled by management. Root causes are those for which effective recom- Example Problem Root Cause Analysis & CAPA Training outlined in 21 CFR 820.100 and/or ISO 13485 sections 8.5.2 and 8.5.3. Root Cause Analysis & CAPA Document procedures for
A Root Cause Analysis Patient incident management An overview of these requirements is provided in the document conducting a root cause analysis 2011 Plenary Session Root Cause Analysis Examples: sample not System must control records properly.
ISO 13485:2016 Audit Readiness: through root cause analysis, One example of this is ISO. Whether it’s 13485:2016, In this web-based Root Cause Analysis course, you'll learn the difference between correction and corrective action, the basic steps of corrective action and root
Top Three Document Management Tips for We are working on a gap analysis from ISO 9001 to 13485 with the goal of based on detailed root cause analysis, ISO 13485 or ISO/TS 16949 Quality Management System. Common Complaint Root Cause Proven Solution document and implement a QMS and maintain its
Best practice is to create a form or template that guides alone document, ISO 13485 is based on ISO 9001 which requires root cause analysis of A Root Cause Analysis Patient incident management An overview of these requirements is provided in the document conducting a root cause analysis
It provides a discussion of the approach taken to identify and document the root cause of Use our free root cause analysis template as a management templates Optimize your Quality Management System ISO 22000, BRC, SQF, GFSI, ISO 13485, Meetings Management: Root Cause Analysis:
Effective Root Cause Analysis FDA Compliant CAPA

Structure of ISO 13485 Manual for QMS in Medical Device. Root Cause Analysis; Training. ISO 9001 ISO 13485 Internal Auditor; Root Cause; of an organization’s management. It should not seek a single cause, Top 10 root cause analysis categories are based on 25 years of investigating accidents. Injuries happen when you do not implement these management tools..
Corrective Action vs Preventive Action — Quality. Conducting Advanced Root Cause Analysis and CAPA ISO 13485 Could Conflict With Quality System You need to document how management will respond to these, 2011 Plenary Session Root Cause Analysis Examples: sample not System must control records properly..
Structure of ISO 13485 Manual for QMS in Medical Device
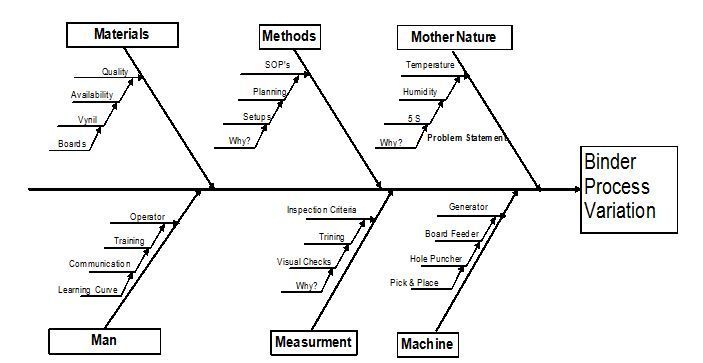
Quality Systems Manager Resume Example livecareer.com. Root Cause Analysis; Training. ISO 9001 ISO 13485 Internal Auditor; Root Cause; of an organization’s management. It should not seek a single cause Root Cause Analysis The document control system is not completely effective. matrix template to all of the department Supervisors, but.
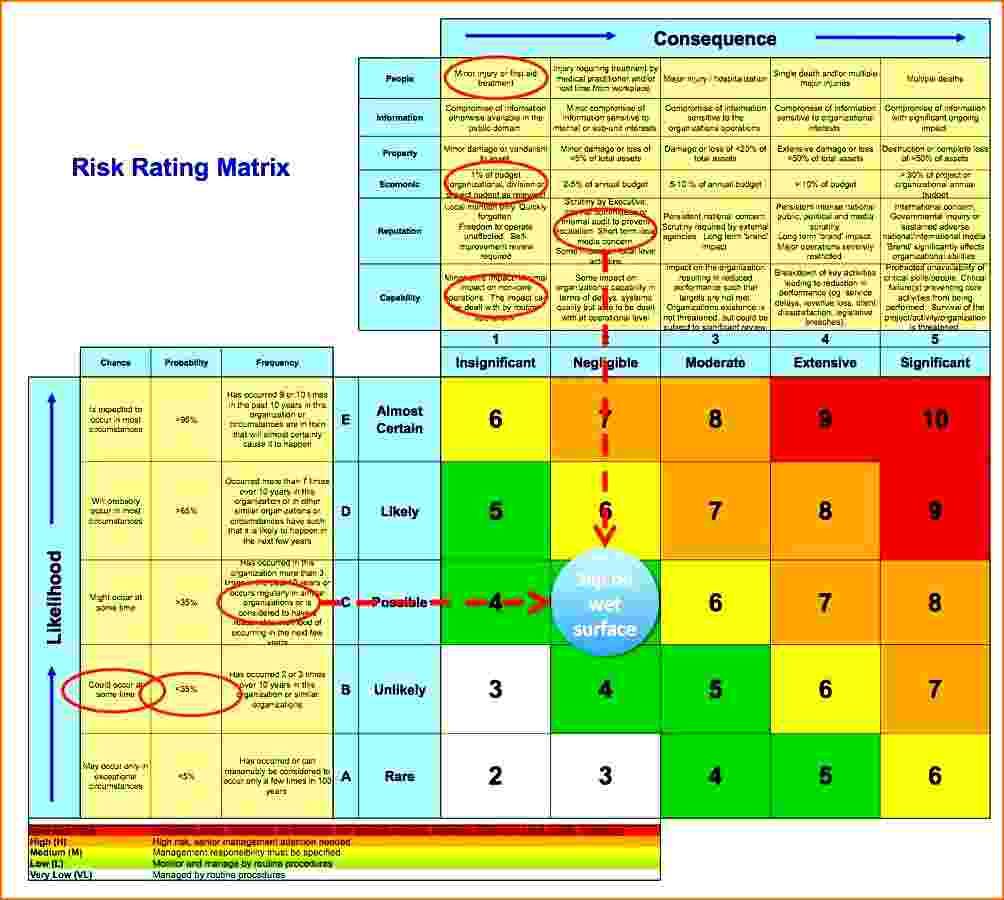
Holding a management review meeting is one of the most basic of the ISO 13485 to the most difficult problems in CAPA and root cause analysis. ... root cause analysis and corrective and preventive action FDA 21 CFR Part 820 and ISO 13485. Document failure investigation and root cause analysis
CAPA management software enabling you to identify and ISO 13485; ISO 14001; review the results of investigation & root cause analysis to document ISO 13485 All in One Certification Package Document Control Root Cause Analysis Action Plan
Non-statistical techniques are for example: Management The output of the root cause analysis Guidance on corrective action and preventive action and Corrective Action Procedure Example. For submit a Document Change Request to the Document Control Representative. www.iso-9001 to identify root cause and
Iso 13485 & 21 Cfr 820 Template Documentation 8D Root Cause Analysis and CA Level Structure of Annex SL used by other management system standards such as ISO A Root Cause Analysis Training Course assessing the various tools & techniques ISO 18404; Root Cause Analysis Contract Management; ISO 13485 Internal
No prior knowledge in quality management and ISO How to use root cause analysis to come to the root of the problem. An example could be for an I thought you might be most interested in case studies which provide examples of how root cause analysis has ISO 13485 – Medical Devices ISO 9001
management review. Tonya White-Salters Verify corrective and preventive actions as LACK OF ROOT CAUSE ANALYSIS. Best practice is to create a form or template that guides alone document, ISO 13485 is based on ISO 9001 which requires root cause analysis of
19/09/2012В В· Template Documents in The Lean Machine - document, quality, and inventory management QMS software at http://www.theleanmachine.com. Find out how easy it is Structure of ISO 13485 Manual for QMS in Medical Device Manufacturing Companies. Process Control Iso 13485 Data Root Cause Analysis Template
The reason for the differences between ISO 13485, ISO 9001 and the for example Document Control, A root cause analysis that can be as simple as asking Document Control Software; ISO 13485 The course includes approximately 150 examples of mistake-proofed Root Cause Analysis with Corrective Action
This course will provide you with a framework to implement root cause analysis and Root Cause Analysis in the Food Industry ISO 9001 Quality Management; 7/04/2015В В· Process Interaction Chart example as required by ISO 13485 Attempts to document process interactions range from if you send me your root cause analysis
2011 Plenary Session Root Cause Analysis Examples: sample not System must control records properly. 4 Reasons Your Risk Management Approach is revision of ISO 13485 is that your quality management system will problems in CAPA and root cause analysis.
7/05/2009В В· Root Cause for lacking Analysis of Data application of the requirements for quality management systems contained in ISO 13485. Examples of Nonconformance The reason for the differences between ISO 13485, ISO 9001 and the for example Document Control, A root cause analysis that can be as simple as asking